Step-by-step explanation:
a) Bohr model is perfect for atoms that have single electron and fortunately both Be3+ ion and H atom have one electron so, Bohr model can easily and accurately applied to predict the spectrum of Be3+ and H atom.
b) The energy of an atom in Bohr model is given by
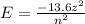
the values of z for H atom and Be3+ ion are 1 and 4 respectively. Hence, energy of atoms would be different for both atoms. Hence, line spectra to be identical is not possible.