Answer:
The order of the energy of the photons of given wave will be
= Ultraviolet waves > infrared waves > microwaves
Step-by-step explanation:
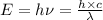
where,
E = energy of photon
 = frequency of the radiation
= frequency of the radiation
h = Planck's constant =
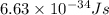
c = speed of light =
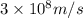
 = wavelength of the radiation
= wavelength of the radiation
We have :
(a) Frequency of infrared waves =
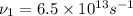
(b) Frequency of microwaves=
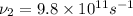
(c) Frequency of ultraviolet waves =
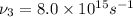
So, the decreasing order of the frequencies of the waves will be :
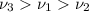
As we can see from the formula that energy is directly proportional to the frequency of the wave.
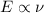
So, the order of the energy of the photons of given wave will be same as their order of frequencies:
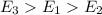
= Ultraviolet waves > infrared waves > microwaves