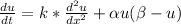Answer:
Explanation:
Given:
Rate k = αμ ( β - μ )
Find:
Derive an equation for the concentration u(x,t)
Solution:
- From the law of conservation we have:
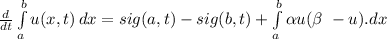
- After dividing the above expression by A i.e cross sectional area of the rod:
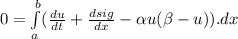
- This is valid for any for any interval [ a, b ] , and the integral is 0:
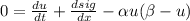
- Now use Fick's law we will obtain a PDE only as a function of u: