We can apply Charles' law to find the change in the volume of the gas since the pressure in this chamber is kept constant.
Step-by-step explanation:
- Charles' law states that at constant pressure, the change in temperature in a chamber will lead to change in its volume, which is to say that temperature and volume changes are directly proportional.
- The volume change here says that the gas expands as the temperature rises, give that the moles of the gas are constant.
- Hence, the equation could be,
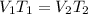 So, Charles' law can be directly applied to the situation to find or analyse the changing the variables such as volume and temperature
So, Charles' law can be directly applied to the situation to find or analyse the changing the variables such as volume and temperature