Answer : The enthalpy of neutralization for this process is, 52.02 kJ/mole
Explanation :
First we have to calculate the moles of HCl and NaOH.
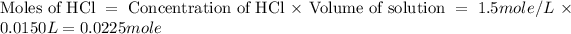
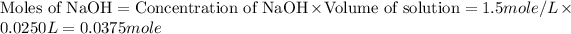
The balanced chemical reaction will be,

From the balanced reaction we conclude that,
As, 1 mole of HCl neutralizes by 1 mole of NaOH
So, 0.0225 mole of HCl neutralizes by 0.0225 mole of NaOH
Thus, the number of neutralized moles = 0.0225 mole
Now we have to calculate the mass of water.
As we know that the density of water is 1 g/ml. So, the mass of water will be:
The volume of water =
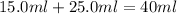
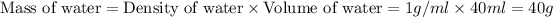
Now we have to calculate the heat absorbed during the reaction.
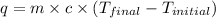
where,
q = heat absorbed = ?
 = specific heat of water =
= specific heat of water =
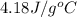
m = mass of water = 40 g
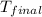 = final temperature of water =
= final temperature of water =
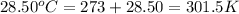
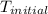 = initial temperature of metal =
= initial temperature of metal =
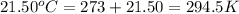
Now put all the given values in the above formula, we get:
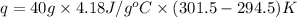
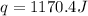
Thus, the heat released during the neutralization = -1170.4 J
Now we have to calculate the enthalpy of neutralization.
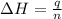
where,
 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?
q = heat released = -1170.4 J
n = number of moles used in neutralization = 0.0225 mole
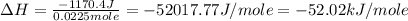
The negative sign indicate the heat released during the reaction.
Therefore, the enthalpy of neutralization for this process is, 52.02 kJ/mole