Answer:
second-law efficiency = 62.42 %
Step-by-step explanation:
given data
temperature T1 = 1200°C = 1473 K
temperature T2 = 20°C = 293 K
thermal efficiency η = 50 percent
solution
as we know that thermal efficiency of reversible heat engine between same temp reservoir
so here
efficiency ( reversible ) η1 = 1 -
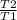 ............1
............1
efficiency ( reversible ) η1 = 1 -
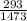
so efficiency ( reversible ) η1 = 0.801
so here second-law efficiency of this power plant is
second-law efficiency =
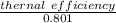
second-law efficiency =
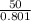
second-law efficiency = 62.42 %