Answer:
A bent molecule with the same length of chemical bonds
Step-by-step explanation:
Ozone has three 3 oxygen atoms and its molecular formula of ozone is
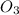 .
.
The central oxygen atom is sp2 hybridized and has one lone pair. Because sp2 hybridization and lone pair, shape is bend. Resonance occur in ozone because lone pair and double bond are in conjugation, therefore, ozone can be represented as a resonance hybrid with same bond lengths.
Therefore, option 2 is correct.
A bent molecule with the same length of chemical bonds