Answer : The correct option is, (d) trigonal bipyramidal
Explanation :
(a) Linear
As we are given the molecular geometry of the molecule is linear and the hybridization of linear molecule is,
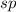 .
.
That means there is 1 atom around the central atom that means one neighboring atom will be occupied by bond pair of electrons and there is no lone pair. Thus, the bond angle between the bond pair and the bond pair will be,
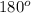
(b) Planar triangular
As we are given the molecular geometry of the molecule is planar triangular and the hybridization of planar triangular molecule is,
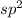 .
.
That means there are 2 atoms around the central atom that means all the neighboring atom will be occupied by bond pair of electrons and there is no lone pair. Thus, the bond angle between the bond pair and the bond pair will be,
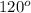
(c) Tetrahedral
As we are given the molecular geometry of the molecule is tetrahedral and the hybridization of tetrahedral molecule is,
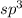 .
.
That means there are 4 atoms around the central atom that means all the neighboring atom will be occupied by bond pair of electrons and there is no lone pair. Thus, the bond angle between the bond pair and the bond pair will be,
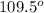
(d) Trigonal bipyramidal
As we are given the molecular geometry of the molecule is trigonal bipyramidale and the hybridization of trigonal bipyramidale molecule is,
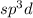 .
.
That means there are 5 atoms around the central atom that means all the neighboring atom will be occupied by bond pair of electrons and there is no lone pair. Thus, the bond angle between the bond pair and the bond pair will be,
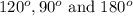
(e) Octahedral
As we are given the molecular geometry of the molecule is octahedral and the hybridization of octahedral molecule is,
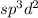 .
.
That means there are 6 atoms around the central atom that means all the neighboring atom will be occupied by bond pair of electrons and there is no lone pair. Thus, the bond angle between the bond pair and the bond pair will be,
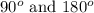
From this we conclude that trigonal bipyramidal has three different values can be observed for the bond angles.
Thus, the correct option is, (d) trigonal bipyramidal