Answer : The amount of in kilojoules released is, -5.53 kJ
Explanation :
Formula used :
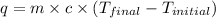
where,
q = heat released = ?
c = specific heat of ethanol =
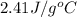
m = mass of ethanol = 23.4 g
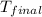 = final temperature =
= final temperature =
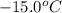
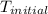 = initial temperature =
= initial temperature =
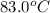
Now put all the given values in the above formula, we get:
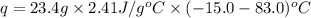
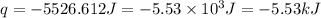
Therefore, the amount of in kilojoules released is, -5.53 kJ