Answer : The concentration of
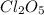 in the vessel 0.820 seconds later is, 0.16 M
in the vessel 0.820 seconds later is, 0.16 M
Explanation :
The given reaction is:
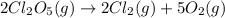
The rate law expression is:
![rate=(6.48M^(-1)s^(-1))[Cl_2O_5]^2](https://img.qammunity.org/2021/formulas/chemistry/college/o0g6ipbzz3zrj2h01yh1xyujxcp147m520.png)
The expression used for second order kinetics is:
![kt=(1)/([A_t])-(1)/([A_o])](https://img.qammunity.org/2021/formulas/physics/college/tlg6nth7imx9pwjb4oksbydlkeyozanniz.png)
where,
k = rate constant =
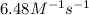
t = time = 0.820 s
![[A_t]](https://img.qammunity.org/2021/formulas/chemistry/high-school/c6se0yk0a5jz0ud2m1a9jh5tv0rk9jx59i.png) = final concentration = ?
= final concentration = ?
![[A_o]](https://img.qammunity.org/2021/formulas/physics/college/3jrctnxyrdjmiz9ngr0s6o9r3hdvpo6qhe.png) = initial concentration = 1.16 M
= initial concentration = 1.16 M
Now put all the given values in the above expression, we get:
![6.48* 0.820=(1)/([A_t])-(1)/(1.16)](https://img.qammunity.org/2021/formulas/chemistry/college/1wu78ntt6l8a4ofbk1c0qzjikqy0ig1vhr.png)
![[A_t]=0.16M](https://img.qammunity.org/2021/formulas/chemistry/college/ln83siyrq52nogfujp97s3zvjztotjmc1b.png)
Therefore, the concentration of
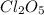 in the vessel 0.820 seconds later is, 0.16 M
in the vessel 0.820 seconds later is, 0.16 M