Answer:
2.1 M is the molarity of the HCl solution.
Step-by-step explanation:

Molarity of HCl solution =
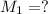
Volume of HCl solution =
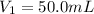
Ionizable hydrogen ions in HCl =
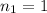
Molarity of NaOH solution =
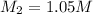
Volume of NaOH solution =
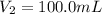
Ionizable hydroxide ions in NaOH =
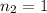
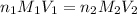 (neutralization )
(neutralization )
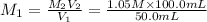
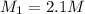
2.1 M is the molarity of the HCl solution.