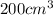The pressure
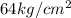 has to be applied to have a volume of
has to be applied to have a volume of
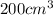
Step-by-step explanation:
According to Boyle's law, for a fixed amount of gas, the volume and pressure are inversely proportional.
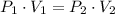
Substituting the values, we get,
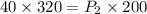
Dividing both sides by 200,
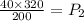
Simplifying the terms, we get,
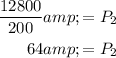
Thus, the pressure
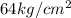 has to be applied to have a volume of
has to be applied to have a volume of