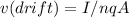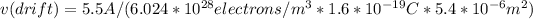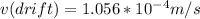Answer:
Drift speed of electrons will be 1.056x10^-4 m/s
Step-by-step explanation
Given Data:
A(area)= 5.4 x 10^-6
I(current)= 5.5 A
Density= 2.7
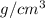
Calculation:
The equation for drift velocity is:
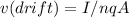
In this case 'q' will be charge of electron which is= 1.6 x 10-19
As each atoms supplies one conduction electron, so number of conduction electrons will be equal to number of atoms.
Hence,
n= no. of conduction electrons/
 = no. of atoms/
= no. of atoms/

To find 'n' we can use following equation:
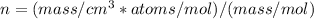
We know atoms/mol is equal to Avogadro`s number i.e 6.02 x 10^23
and molar mass of aluminium is 26.982 g.
Now,
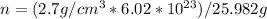 (putting values in above equation)
(putting values in above equation)
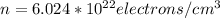
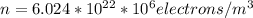 (converting electrons/cm3 to electrons/m3)
(converting electrons/cm3 to electrons/m3)
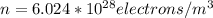
To find drift velocity, we will use equations mention before: