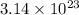Answer : The number of moles of ions form are,
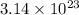 and there are 3 ions formed.
and there are 3 ions formed.
Explanation :
First we have to calculate the moles of calcium iodide.
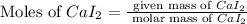
Molar mass of calcium iodide = 293.9 g/mol
Mass of calcium iodide = 51.0 g
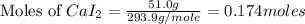
Now we have to calculate the number of moles of ions.
As we know that when calcium iodide dissolved in water then it dissocites to give calcium ion and iodide ion.
The balanced chemical reaction will be:
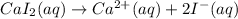
From this we conclude that there are 3 ions formed.
As, 1 mole of calcium iodide dissociate to give
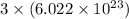 number of ions
number of ions
So, 0.174 mole of calcium iodide dissociate to give
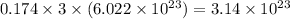 number of ions
number of ions
Thus, the number of moles of ions form are,