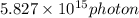Answer:
number of photon will be equal to
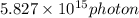
Step-by-step explanation:
We have given wavelength of nitrogen laser pulse is 337 nm
So wavelength
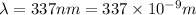
Velocity of light
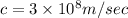
Plank's constant
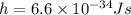
So energy of each photon
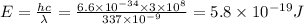
Total energy is given = 3.38 mJ = 0.00338 J
So number of photon will be equal to
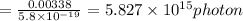
So number of photon will be equal to