Answer:
The extinction coefficient is 1.15 x 10⁴ M⁻¹.cm⁻¹ (value is not rounded off)
Step-by-step explanation:
According to Lambert-Beer law
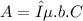
Here, A is absorbance, ε is extinction coefficient, b is the length of the cuvette and C is the molar concentration of substance X.
This equation is used for the relation between concentration and absorbance of electromagnetic radiation absorbing species. It is a linear equation and can be used for making a calibration curve, which is used for the analysis of an unknown concentration solution. The slope of this curve according to the equation is the product of extinction coefficient (M⁻¹.cm⁻¹) and the length of the cuvette in cm.
In this problem, the slope is provided, which can be mathematically represented as:
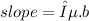
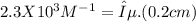
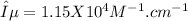 (not rounded off)
(not rounded off)
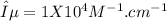 (rounded off)
(rounded off)