Answer:
The amount of Cl2 gas left , after the reaction goes to completion is : 139.655 grams
Step-by-step explanation:
Molar mass : It is the mass in grams present in one mole of the substance.
Moles of the substance is calculated by:
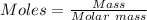
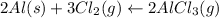
According to this equation:
2 mole of Al = 3 mole of Cl2 = 2 mole of AlCl3
Molar mass of Al = 27.0 g/mol
Mass of Al = 20.1 gram
Moles of Al present in the reaction :
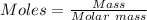
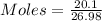
Moles of Al = 0.744
Similarly calculate the moles of Cl2
Molar mass of Cl2 = 71.0 g/mol
Mass = 219 gram
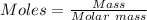
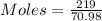
Moles of Cl2 = 3.08 moles
According to equation,
2 mole of Al reacts with = 3 mole of Cl2
1 moles of Al reacts with = 3/2 mole of Cl2
0.744 moles of Al reacts with = 3/2(0.744) moles of Cl2
= 1.116 moles of Cl2
But actually present Cl2 = 3.08 moles
Hence Al is the limiting reagent , and Cl2 is the excess reagent.
The whole Aluminium Al get consumed during the reaction.
The amount of Cl2 in excess = Total Cl2 - Cl2 consumed
Cl2 in excess = 3.08 - 1.116 = 1.964 moles
Cl2 in grams = 1.964 x 70.90 = 139.655 grams