Answer:
4. 1; 6; 2 — synthesis
Step-by-step explanation:
Decomposition reaction is defined as the reaction in which a single large substance breaks down into two or more smaller substances.
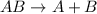
Single displacement reaction is defined as the reaction in which more reactive element displaces a less reactive element from its chemical reaction.
The reactivity of metal is determined by a series known as reactivity series. The metals lying above in the series are more reactive than the metals which lie below in the series.
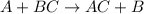
Synthesis reaction is defined as the reaction in which smaller substances combine in their elemental state to form a larger substance.
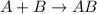
The unbalanced combustion reaction is shown below as:-
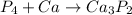
On the left hand side,
There are 4 phosphorus atoms and 1 calcium atom
On the right hand side,
There are 2 phosphorus atoms and 3 calcium atoms
Thus,
Right side,
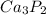 must be multiplied by 2 to balance phosphorus.
must be multiplied by 2 to balance phosphorus.
Left side,
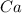 is multiplied by 6 so to balance the whole reaction.
is multiplied by 6 so to balance the whole reaction.
Thus, the balanced reaction is:-
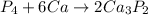
Thus, answer:- 4. 1; 6; 2 — synthesis