Answer: IR-A possess more energy than IR-C
Step-by-step explanation:
Before anything, you need to understand that; the longer the wavelength a wave possess, the smaller the energy it has.
We have to use planck's equation and C=λv
where; C = Speed of light (3.0 x
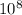 m/s)
m/s)
λ = wave length
v = frequency
planck's equation = E = hv
Equating the two equations; we get
E/h = C/λ
E = Ch/λ
where;
h = planck's constant (6.63 x
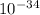 Js)
Js)
Comparing the energies of microwaves,
FOR IR-A
when
λ = 700nm = 700 x
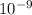 m
m
E = Ch/λ
E =
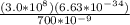
E = 2.84 x
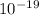 J
J
when
λ = 1400nm = 1400 x
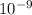 m
m
E = Ch/λ
E =
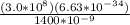
E = 1.42 x
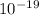 J
J
FOR IR-C
when
λ = 3000nm = 3000 x
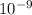 m
m
E = Ch/λ
E =
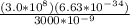
E = 6.63 x
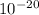 J
J
when
λ = 1,000,000nm = 1,000,000 x
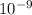 m
m
E = Ch/λ
E =
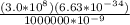
E = 1.99 x
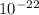 J
J