Answer:
The answer to your question is pH = 2.28
Step-by-step explanation:
Chemical reaction
HCOOH(ac) + H₂O ⇔ H₃O⁺ + HCOO⁻
I 0.15 -- 0 0
C - x -- +x +x
F 0.15 - x -- x x
Write the equation of equilibrium
![ka = ([H3O][HCOO])/([HCOOH])](https://img.qammunity.org/2021/formulas/chemistry/college/u9p16f56s1reyucw4z7qzji2odkuj6pq44.png)
Substitution
![1.8 x 10^(-4) = ([x][x])/(0.15 - x)](https://img.qammunity.org/2021/formulas/chemistry/college/ct40vttihdpfogej1ujw1bs1w5e9ymnfrf.png)
But 0.15 - x ≈ 0.15
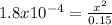
Solve for x
x² = (0.15)(1.8 x 10⁻⁴)
Simplification
x² = 0.000027
Result
x = 0.0052
pH = -log [H₃O⁺]
Substitution
pH = - log [0.0052]
Simplification and result
pH = 2.28