Answer: (a). W = 4KJ and Q = 4.25KJ
(b). W = -2KJ and ΔPE = 2KJ
Step-by-step explanation:
(a).
i. We are asked to calculate the work done during the expansion process considering gas as system.
from W =
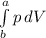 where a = V₂ and b = V₁
where a = V₂ and b = V₁
so W = P(V₂-V₁)
W = (2 × 10²) (0.12 - 0.10)
W = 4 KJ
ii. We apply the energy balance to gas as system
given Q - W = ΔE
Where ΔE = ΔU + ΔKE + ΔPE
since motion of the system is constrained, there is no change in both the potential and kinetic energy i.e. ΔPE = ΔKE = 0
∴ Q - W = ΔU
Q = ΔU + W
Q = 0.25 + 4
Q = 4.25 KJ
(b).
i. to calculate the work done during the expansion process considering piston as system;
W =
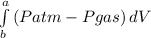 where a and b represent V₂ and V₁ respectively.
where a and b represent V₂ and V₁ respectively.
W = (Patm - Pgas)(V₂ - V₁)
W = (1-2) ×10² × (0.12-0.1)
W = -2KJ
ii. We apply the energy balance to gas as system
given Q - W = ΔE
Where ΔE = ΔU + ΔKE + ΔPE
Q = 0 since the piston and cylinder walls are perfectly insulated.
for piston, we neglect the change in internal energy and kinetic energy
ΔU = ΔKE = 0
from Q - W = ΔU + ΔKE + ΔPE
0 - (-2) = 0 + 0 + ΔPE
∴ ΔPE = 2KJ