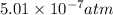The question is incomplete, here is the complete question:
Consider this reaction occurring at 298 K:
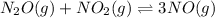
If a reaction mixture contains only
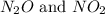 at partial pressures of 1.0 atm each, the reaction will be spontaneous until some NO forms in the mixture.
at partial pressures of 1.0 atm each, the reaction will be spontaneous until some NO forms in the mixture.
What maximum partial pressure of NO builds up before the reaction ceases to be spontaneous. Given that:
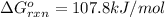
Answer: The maximum partial pressure of NO will be
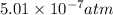
Step-by-step explanation:
For the given chemical equation:
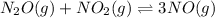
The expression of
 for above equation follows:
for above equation follows:
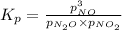
When the reaction ceases to be spontaneous, the
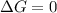 (at equilibrium)
(at equilibrium)
Relation between standard Gibbs free energy and equilibrium constant follows:
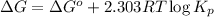
where,
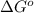 = Standard Gibbs free energy = 107.8 kJ/mol = 107800 J/mol (Conversion factor: 1 kJ = 1000 J )
= Standard Gibbs free energy = 107.8 kJ/mol = 107800 J/mol (Conversion factor: 1 kJ = 1000 J )
R = Gas constant =
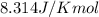
T = temperature = 298 K
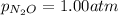
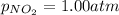
Putting values in above equation, we get:
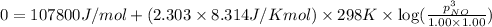
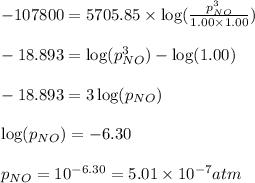
Hence, the maximum partial pressure of NO will be