Answer:

Step-by-step explanation:
2Al(s) + Fe₂O₃(s) ⟶ Al₂O₃(s) + 2Fe(s); ΔᵣH = ?
The formula for calculating the enthalpy change of a reaction by using the enthalpies of formation of reactants and products is
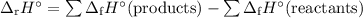
2Al(s) + Fe₂O₃(s) ⟶ Al₂O₃(s) + 2Fe(s)
ΔfH°/kJ·mol⁻¹: 0 -824.3 -1675.7 0
![\begin{array}{rcl}\Delta_{\text{r}}H^(\circ) & = & [1(-1675.7) + 2(0)] - [2(0) - 1(-824.3)]\\& = & -1675.7 + 824.3\\& = & \textbf{-851.4 kJ/mol}\\\end{array}\\\text{The enthalpy change is } \large \boxed{\textbf{-851.4 kJ/mol}}](https://img.qammunity.org/2021/formulas/chemistry/middle-school/ah8rnraf9x783wmljbnahxi55ynpezkpg4.png)