Answer: The ratio of f at the higher temperature to f at the lower temperature is 4.736
Step-by-step explanation:
According to the Arrhenius equation,
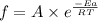
or,
![\log ((f_2)/(f_1))=(Ea)/(2.303* R)[(1)/(T_1)-(1)/(T_2)]](https://img.qammunity.org/2021/formulas/physics/high-school/s65mqyle81qh6lmcvpy97yd0ay2tncvs2x.png)
where,
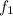 = rate constant at 525K
= rate constant at 525K
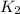 = rate constant at 545K
= rate constant at 545K
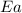 = activation energy for the reaction = 185kJ/mol= 185000J/mol (1kJ=1000J)
= activation energy for the reaction = 185kJ/mol= 185000J/mol (1kJ=1000J)
R = gas constant = 8.314 J/mole.K
 = initial temperature = 525 K
= initial temperature = 525 K
 = final temperature = 545 K
= final temperature = 545 K
Now put all the given values in this formula, we get
![\log ((f_2)/(f_1))=(185000J/mol)/(2.303* 8.314J/mole.K)[(1)/(525K)-(1)/(545K)]](https://img.qammunity.org/2021/formulas/physics/high-school/2msb21s8oddc8qkhy4an0q7d4lt482gejg.png)
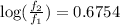
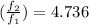
Therefore, the ratio of f at the higher temperature to f at the lower temperature is 4.736