Answer: The number of electrons in the given amount of silver are

Step-by-step explanation:
To calculate the number of moles, we use the equation:

Given mass of silver = 9.0 g
Molar mass of silver = 107.87 g/mol
Putting values in above equation, we get:
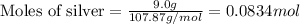
We are given:
Number of electrons in one atom of silver = 47
According to mole concept:
1 mole of an element contains
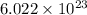 number of particles
number of particles
So, 0.0834 moles of silver contains
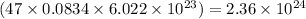 number of electrons
number of electrons
Hence, the number of electrons in the given amount of silver are
