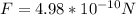Answer:
Step-by-step explanation:
We have a sodium cation, that is, an ion with a positive charge and a chloride anion, that is, an ion with a negative charge. According to Coulomb's law, the magnitude of the electrostatic force is given by:
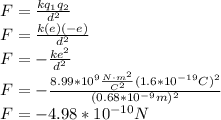
The negative sign indicates that is an attractive force. So, the magnitude of the force is: