Step-by-step explanation:
As
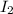 is a covalent compound because it is made up by the combination of two non-metal atoms. Atomic number of an iodine atom is 53 and it contains 7 valence electrons as it belongs to group 17 of the periodic table.
is a covalent compound because it is made up by the combination of two non-metal atoms. Atomic number of an iodine atom is 53 and it contains 7 valence electrons as it belongs to group 17 of the periodic table.
Therefore, sharing of electrons will take place when two iodine atoms chemically combine with each other leading to the formation of a covalent bonding.
Hence, weak forces like london dispersion forces will be present between a molecule of
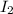 .
.
The weak intermolecular forces which can arise either between nucleus and electrons or between electron-electron are known as dispersion forces. These forces are also known as London dispersion forces and these are temporary in nature.
thus, we can conclude that london dispersion force is the major attractive force that exists among different
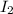 molecules in the solid.
molecules in the solid.