Answer:
1. 19 moles of P
2. Grams of Phosphorous produced = 15.91 grams
Step-by-step explanation:
The balanced equation is :
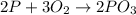
PO3 = phosphorous trioxide
O2 = Oxygen
P = phosphorous
1. Here according to stoichiometry:
2 moles of P = 3 moles of O2 = 2 moles of PO3
Or ,
2 moles of PO3 is produced from = 2 moles of P
So, 1 mole of PO3 is produced from = 1 mole of P
19 moles of PO3 is produced from = 1 x19 = 19 moles of P
Hence , Moles of P=
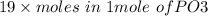
= 19 moles of P
2. Molar mass of P = 31 grams
Or 1 mole of P = 31 grams
3 mole of P = 3 x 31 = 93 gram
(whether we write 1 mole or 31 gram , it is same thing )
Again , from stoichiometry of the balanced equation,
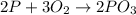
3 moles of O2 reacts with = 2 moles of P
1 mole of O2 reacts with =
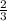 of P
of P
0.77 moles of O2 reacts with=
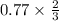 of P
of P
Always calculate for 1 mole first, then multiply by the given number
= 0.513 moles of P
Mass of 1 mole P = 31 gram
Mass of 0.5133 mole of P = 0.5133 x 31 gram
= 15.9 grams (16 grams)