Answer:
The mass of lead(ll)tetraoxosuplhate formed when 0.5mol-¹ of tetraoxosulphate react with lead(ll)chloride is :
151.63 grams
Step-by-step explanation:
PbCl2 = lead(ll)chloride
PbSO4 = lead(ll)tetraoxosuplhate
H2SO4 = hydrogen tetraoxosulphate
HCl = Hydrochloric acid
The balanced equation for the reaction is :
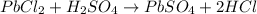
The stoichiometric coefficients indicates that :
1 mole PbCl2 = 1 mole H2SO4 = 1 mole PbSO4 = 2 mole HCl
1 mole H2SO4 produces = 1 mole PbSO4
0.5 mole produce = 0.5 mole of PbSO4
We have 0.5 mole of PbSO4
So , we are asked to calculate the mass of 0.5 mole pf PbSO4
Molar mass of PbSO4 = mass of Pb + Mass of S + 4(mass of O)
= 207.2 + 32.06 +4(16)
=303.26 grams
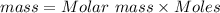
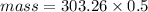
151.63 grams