Answer:
III. lithium nitrate, LiNO₃
II. 1-butanol
IV. ethane, C₂H₆.
I. methane
Step-by-step explanation:
The solubility of the substances are influenced by many factors. One of the factors are whether the substances are salts or not salts.
Now, metal salts are more soluble in water. This is because of their ability to form aqueous solutions in a liquid medium. For example, a metal salt HX dissolves in water to give:
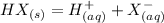
This results in an ionic solution
1-butanol follows next because it is an alcohol. Alcohols form hydrogen bonds with water but are not strongly ionic as metal salts
Ethane, C₂H₆. forms weak dipole associations in water. This is because of the electron cloud on the double carbon bond, C=C
Methane forms very weak spontaneous associations and is not very soluble in water. It is the least soluble