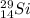Answer : The isotopic representation of silicon atom is:
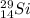
Explanation :
Isotope : It is defined as the element that have the same number of protons but have the different number of neutrons of each of the atom.
Isotope notation or nuclear symbol : It is also called as nuclear notation. In isotopic notation we can easily determine an isotope's mass number, atomic number, the number of neutrons and protons in the nucleus.
The general isotopic representation of an atom is:
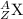
where,
Z = Atomic number of the atom
A = Mass number of the atom
X = Symbol of the atom
As we are given that the atom is, silicon.
Atomic number of Si atom = 14
Mass number of Si atom = 29
The symbol of silicon = (Si)
The isotopic representation of silicon atom is: