This is an incomplete question, here is a complete question.
Hydrogen chloride and oxygen react to form water and chlorine, like this:
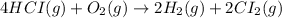
Use this chemical equation to answer the questions in the table below.
Suppose 155. mmol of HCl and 38.8 mmol of O₂ are added to an empty flask. How much HCl will be in the flask at equilibrium?
(1) None.
(2) Some, but less than 155. mmol,
(3) 155. mmol,
(4) More than 155. mmol,
Answer : The correct option is, (1) None
Explanation :
The given balanced chemical reaction is:
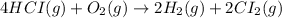
From the balanced chemical reaction, we conclude that
As, 4 mmole of HCl react with 1 mmoles of

So, 155. mmole of HCl react with
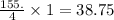 mmoles of
mmoles of

That means,
 is excess reagent and
is excess reagent and
 is a limiting reagent. All the moles of HCl will consume.
is a limiting reagent. All the moles of HCl will consume.
Hence, the correct option is, None.