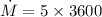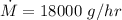Answer
given,
Q₁ = 10,000 J
W = 2000 J
heat of combustion, Lc = 5 x 10⁴ J/g
W = Q₁ - Q₂
Q₂ = Q₁ - W
Q₂ = 10,000 - 2000
Q₂ = 8000 J
Q₂ is the rejected to heat.
a) thermal efficiency of this engine
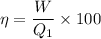
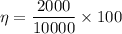
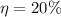
b) discarded heat is equal to 8000 J.
c) Power output
P = N W
N = 25 cycle/s
P = 25 x 2000 = 50000 W
1 W = 746 Horsepower
Power in horsepower
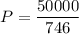
P = 67 HP
d) gasoline burnt is equal to
Q₁ = m Lc
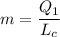
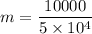
m = 0.2 gram/cycle
e) gasoline burnt per hour
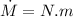
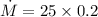
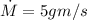
1 Hr = 3600 s