Answer:
Chemical reactions do not involve changes in the chemical bonds that join
atoms in compounds :
False
Step-by-step explanation:
Chemical reaction are the reaction in which old bonds break and new bonds are formed . The formation of new bond result in formation of new compounds . This happen because new bond are result of linking different atoms by the bond.
For example : Water formation from Oxygen and Hydrogen is a chemical process :
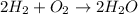
Original(old) bonds are :
H-H bond in H2 and O-O bonds in O2
In H2 = Hydrogen is joined to Hydrogen
IN O2 = Oxygen is joined to oxygen
New Bonds =
O-H bonds in water (H2O)
Oxygen is joined to hydrogen = New Bond formation
Hence,
Chemical reactions do involve changes in the chemical bonds that join
atoms in compounds