Answer:
The answer to your question is 4.2 l
Step-by-step explanation:
Data
Temperature 1 = T1 = 15°C = 288ºK
Pressure 1 = P1 = 2 atm
Volume 1 = V1 = 2 l
Temperature 2 = T2 = 30°C = 303ºK
Pressure 2 = P2 = 1 atm
Volume 2 = V2 = ?
Formula
Combined gas law
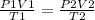
Solve for V2
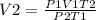
Substitution
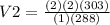
Simplification
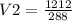
Result
V2 = 4.2l