Answer: single replacement
Step-by-step explanation:
a) Synthesis reaction is a type of chemical reaction in which two reactants combine to give a single product.
b) Decomposition reaction is a type of chemical reaction in which one reactant give two or more than two products.
c) A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution. Thus one element should be different from another element.
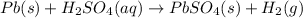
d) A double displacement reaction is one in which exchange of ions take place. The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.