Answer:
It is added 250J of energy as heat.
Step-by-step explanation:
First law of thermodynamics relates internal energy (
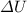 ), work (W) and heat (Q) of a system in the next way:
), work (W) and heat (Q) of a system in the next way:
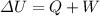
Because we already know
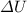 and W values, the only thing we should do to find the energy removed (sign of Q negative) or added (sign of Q positive) as heat is solve (1) for Q:
and W values, the only thing we should do to find the energy removed (sign of Q negative) or added (sign of Q positive) as heat is solve (1) for Q:
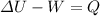
Work is negative because is done by the gas to the surroundings, so W= -50J and
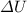 is positive becuse it increases, then:
is positive becuse it increases, then:
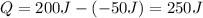
Because the sign of Q is positive heat is added to the engine.