Answer:
The balance chemical equation of combustion of methane is:
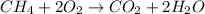
Step-by-step explanation:
[tex]CH_4 + O_2\rightarrow CO_2 + H_2O[/tex]
During balancing of the chemical reaction balance out all the elements beside oxygen. Oxygen atom should always be balanced in the end.
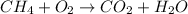
On reactant side :
C = 1, H = 4, O = 2
On product side :
C = 1, H = 2, O = 3
Step 1 : Write 2 in front of water.
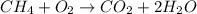
Step 2 : Write 3 in front of oxygen.
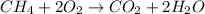
On reactant side :
C = 1, H = 4, O = 4
On product side :
C = 1, H = 4, O = 4
Chemical equation is balanced.