Answer:
168 grams of O2 are produced from 356.6g of Al2O3
Step-by-step explanation:
Calculate the moles of Al2O3
Given mass = 356.6
Molar mass of Al2O3 = 2(mass of Al) +3(mass of O)
= 2(27) + 3(16)
=102 g/mol
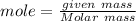
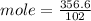
= 3.5 mole
The balanced equation for the equation is :
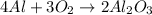
2 mole of Al2O3 will give 3 mole of Oxygen
1 mole of Al2O3 = 3/2 = 1.5 mole of Oxygen
3.5 mole of Al2O3 = (1.5 x 3.5 ) mole of Oxygen
3.5 mole of Al2O3 = 5.25 moles of oxygen
Use,
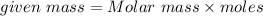
Molar mass of O2 = 16(2) = 32 g/mol
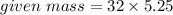
= 168 g