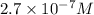Answer : The concentration in (M)of bromide ions in a saturated solution of mercury (II) bromide is,
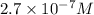
Explanation :
The solubility equilibrium reaction will be:
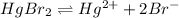
Let the molar solubility be 's'.
The expression for solubility constant for this reaction will be,
![K_(sp)=[Hg^(2+)][Br^(-)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/eg7m5ybdfxhrtd3vqgf09dddhjrt2e2ber.png)
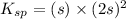
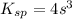
Given:
 =
=
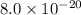
Now put all the given values in the above expression, we get:
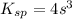
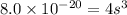

Therefore, the concentration in (M)of bromide ions in a saturated solution of mercury (II) bromide is,