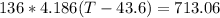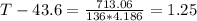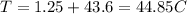Answer:
44.85C
Step-by-step explanation:
Let the specific heat of glass thermometer be 0.84 J/g°C
Let the specific heat of water be 4.186 j/g °C
Let the water density be 1kg/L
136 mL of water = 0.136L of water = 0.136 kg of water = 136 g of water
Since the change of temperature on the glass thermometer is 43.6 - 22 = 21.6 C. We can then calculate the heat energy absorbed to it:
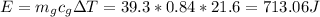
Assume no energy is lost to outside, by the law of energy conservation, this heat energy would come from water