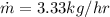Answer:
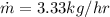
Step-by-step explanation:
Given data:

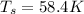
Radius of contaner = 0.589 m
emissivity is = 1
surface area =
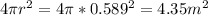
Net Radiation between heilum and shell is given as
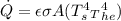
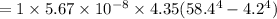
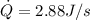
for 6.76 hr = 70,087.68 J/hr
rate of energy removed can be calculated by using the following relation
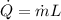
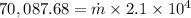
solving for \dot m we get