Answer:
The energy required to increase the temperature of the diatomic ideal gas by 1 degree C is 118.18 kJ.
Step-by-step explanation:
Given that,
Volume = 100 m³
Temperature = 300 K
Suppose Consider heating it at constant pressure.
We need to calculate the energy required to increase the temperature of the diatomic ideal gas by 1 degree C
Using formula of heat energy
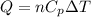
Where, n = numbers of moles of gas
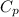 =specific heat capacity
=specific heat capacity
 = change in temperature
= change in temperature
We know that,
The specific heat capacity for diatomic ideal gas
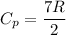
Put the value into the formula of heat
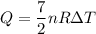
Substitute the value of nR into the formula
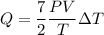
Here, P = pressure of the air
V = volume of the air
Put the value into the formula
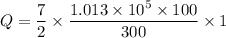
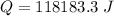
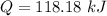
Hence, The energy required to increase the temperature of the diatomic ideal gas by 1 degree C is 118.18 kJ.