Answer:
Silicon dioxide
Step-by-step explanation:
Both silicon and oxygen are nonmetals, meaning we are dealing with a molecular compound.
In order to name this compound, we firstly have to derive its chemical formula. Silicon is in group 4A, so it has 4 valence electrons and an oxidation state of +4. Similarly, oxygen is in group 6A and has a total of 6 valence electrons, an oxidation state of -2.
As a result, it takes two oxygen atoms to balance the oxidation state of silicon and have a neutral compound which yields a chemical formula of
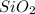 .
.
Naming a molecular compound would require us to use prefixes. We have one silicon atom (mono) and two oxygen atoms (di). We would never use 'mono' for the first element, so we simply end up with silicon dioxide.