Answer:
A) 36 g
Step-by-step explanation:
From the source, some values have been correct.
Given that;-
Mass of NaCl formed = 116 grams
Molar mass of NaCl = 58 g/mol
Moles of NaCl =
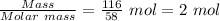
According to the reaction shown below:-

1 mole of NaCl and 1 mole of
 are produced upon reaction.
are produced upon reaction.
So,
2 moles of NaCl and 2 moles of
 are produced upon reaction.
are produced upon reaction.
Moles of water = 2 moles
Molar mass of water = 18 g/mol
Mass =
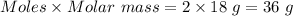
36 g of
 is produced.
is produced.