Question:
1. (NH)2CrO
a) Number of moles of H:
b) Number of moles of N:
Answer:
a) Number of moles of H: 2
b) Number of moles of N: 2
Explanation:
The
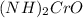 is ammonium Chromate which is monoclinic and yellow Crystal that is formed due to the reaction of ammonium Hydroxide and ammonium di-chromate. It is used as catalyst, corrosion inhibitor as well as analytical inhibitors.
is ammonium Chromate which is monoclinic and yellow Crystal that is formed due to the reaction of ammonium Hydroxide and ammonium di-chromate. It is used as catalyst, corrosion inhibitor as well as analytical inhibitors.
Question:
2. Ag.SO.
a) Molar Mass:
b) Percent Composition of Ag:
c) Percent Composition of S:
d) Percent Composition of O:
Answer:
a) Molar Mass: 155.93 Kg
b) Percent Composition of Ag: 69%
c) Percent Composition of S: 20.5%
d) Percent Composition of O: 10.2%
Step-by-step explanation:
Molar mass = molar mass of Ag + molar mass of S + molar mass of O
=>107.87+32.06+16
=> 155.93 Kg
Percent Composition of Ag
=
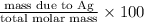
=
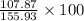
= 0.69 \times 100
= 69%
Percent Composition of S:
=
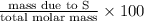
=
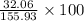
= 0.205 \times 100
= 20.5%
Percent Composition of O:
=
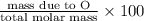
=
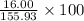
= 0.102 \times 100
= 10.2%