Answer:
time for NO₂ to diffuse = 90 s
Step-by-step explanation:
Data Given:
Amount of O₂ gas transferred = 80 cm³
time of O₂ gas to diffuse = 50s
Amount of NO₂ gas transferred = 120 cm³
time of NO₂ gas to diffuse = ?
Solution:
As we know
rate of diffusion = Amount of gas transferred / time . . . . . . . (1)
we also know that Graham's Law is
rate of diffusion gas A/rate of diffusion gas B =
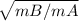 . . . (2)
. . . (2)
where
mA = molar mass of gas A
mB = molar mass of gas B
combine both equation 1 and 2
(Amount of gas A transferred / time for gas A) / (Amount of gas B transferred / time for gas B) =
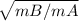 . . . . . . . . (3)
. . . . . . . . (3)
we can write equation 3 for oxygen and nitrogen (iv) oxide
(Amount of gas O₂ transferred / time for gas O₂) / (Amount of gas NO₂ transferred / time for gas NO₂) =
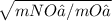 . . . . . . . (4)
. . . . . . . (4)
- molar mass of O₂ = 2 (16) = 32 g/mol
- molar mass of NO₂ = 14 + 2(16) = 46 g/mol
Put values in equation equation 4
(80 cm³/50 s) / (120 cm³ / time for NO₂) =
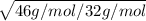
(1.6 cm³/s)/(120 cm³ / time for NO₂) =
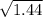
(1.6 cm³/s) / (120 cm³ / time for NO₂) = 1.2
Rearrange the above equation
time for NO₂ = 1.2 /(1.6 cm³/s) x 120 cm³
time for NO₂ = 90 s
So,
time for NO₂ to diffuse = 90 s