Answer:
No. While gold would not react with a silver nitrate solution, nickel would.
Step-by-step explanation:
Refer to the metal reactivity series.
Reactivity:
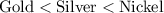 .
.
Gold is positioned after silver in the reactivity series, meaning that gold is typically less reactive than silver. Thus, gold would not react with a solution of silver ions to produce silver metal.
However, since nickel is positioned before silver in the reactivity series, it is expected that nickel would react with silver ions in this solution to produce silver metal.
Thus, if the silver nitrate solution comes into contact with the two rings, the nickel ring would likely react with the solution, the gold ring would not.