Answer:
The third one:
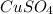
Step-by-step explanation:
Again you are going to want to look at a periodic table so you can determine the symbol of each atom.
The symbol for copper is Cu, the symbol for sulfur is S and the symbol for Oxygen.
There is 1 atom of copper in the compound
So we have Cu
There is 1 atom of sulfur in the compound
So we would have CuS
There are 4 atoms of oxygen in the compound
So we would have
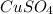
Note that the little number at the bottom left after each symbol indicates how many atoms of that element are in that compound.