Answer:

Step-by-step explanation:
We are asked to calculate the number of moles of water, given the molecules of water.
We will convert moles to molecules using Avogadro's Number or 6.022 × 10²³. This is the number of particles (atoms, molecules, formula units, etc.) in 1 mole of a substance. In this case, the particles are molecules of water or H₂O.
We will convert using dimensional analysis, so we must set up a ratio using Avogadro's Number.
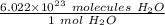
We are converting 3 × 10²² molecules of water to moles, so we must multiply the ratio by this value.
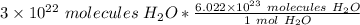
Flip the ratio so the units of molecules of water cancel.
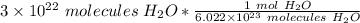
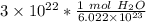
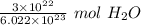
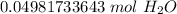
The original value of molecules of water ( 3 × 10²² ) has 1 significant figure, so our answer must have the same number of sig figs. For our answer that is the hundredth place. The 9 in the thousandth place tells us to round the 4 in the hundredth place up to a 5.
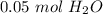
3×10²² molecules of water are equal to 0.05 moles of water.